Biofisika Institute contributes to uncovering the dynamic inner workings of nuclear pore complexes
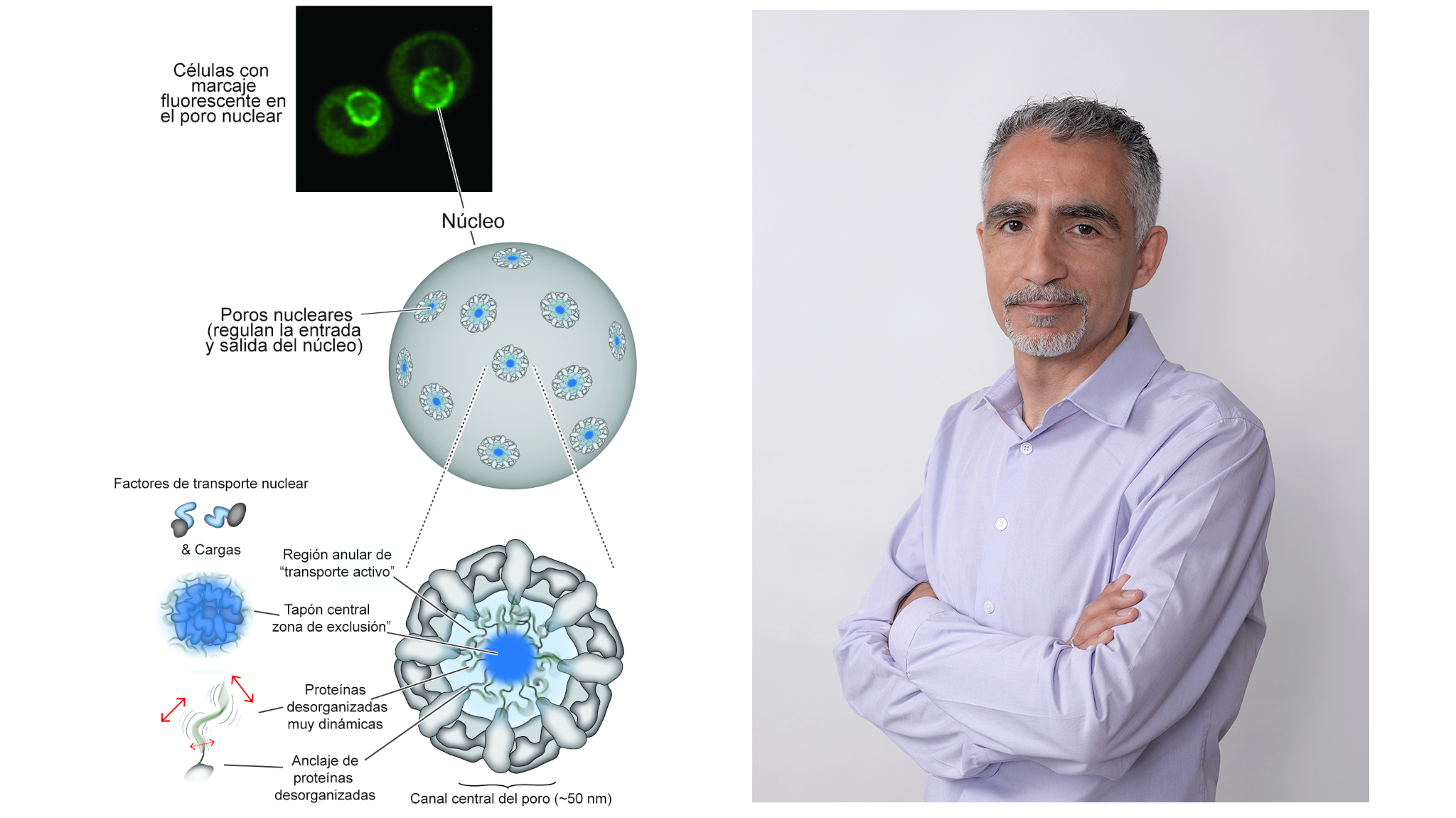
A study published in Nature Cell Biology reveals that these cellular structures, which play a key role in mediating the communication between the nucleus and cytoplasm, are dynamically organized, constantly moving and rearranging.
The Biofisika Institute (CSIC-EHU)-FBB has been a key part of an international study that, for the first time, captures the dynamic behavior of nuclear pore complexes (NPCs) with millisecond precision. These structures, essential for regulating molecular exchange between the nucleus and cytoplasm, were observed in action using high-speed atomic force microscopy. The research, published in Nature Cell Biology, involved the laboratory of Javier Fernández Martínez, Ikerbasque Research Professor at the Biofisika Institute and Biofísica Bizkaia Foundation, alongside scientific teams from Switzerland, the United States, the Netherlands, and Spain.
NPCs are protein complexes that act as selective gateways to the cell nucleus. For decades, their internal structure was thought to function as a static barrier, based on models describing a mesh of proteins resembling a hydrogel. This new study, however, demonstrates that NPCs are highly dynamic and self-organizing structures.
Using high-speed atomic force microscopy (HS-AFM), the team led by Professor Roderick Lim (Biozentrum and Swiss Nanoscience Institute, University of Basel) visualized nanoscopic movements unfolding within milliseconds inside the transport channel of the pore. These observations revealed a mobile “central plug” composed of transport proteins, cargo molecules, and low complexity protein “threads” (FG nucleoporins), which regulates molecular flow and creates distinct zones of passage.
Experimental validation:
The findings were confirmed through complementary in vitro and in vivo experiments. In lab-made DNA origami pores —artificial structures designed to mimic the size and function of NPCs— the same dynamic behavior was observed. In living cells, the team found that a proper balance between FG nucleoporin density and nuclear transport factor binding is essential for pore functionality.
Scientific implications:
This discovery challenges a traditional model of NPCs as hydrogel-like barriers and proposes a unified view that integrates long-standing structural and biochemical observations. NPCs are now understood as adaptive, self-organizing molecular gates, with implications for cell biology, biotechnology, and the development of drug delivery systems.
The participation of the Biofisika Institute in this research reinforces its role as a reference center in the study of molecular mechanisms that regulate cellular organization. As part of the Basque biomedical ecosystem, IBF actively contributes to advancing fundamental knowledge in biophysics and structural biology, and to developing biomedical technologies with applications in diagnostics, targeted therapies, and personalized medicine.
The work is based on an international collaboration between researchers from the Biozentrum and the Swiss Nanoscience Institute at the University of Basel (Switzerland), Rockefeller University (New York, USA), the Basque Foundation for Science (Bilbao, Spain), the University of the Basque Country (Leioa, Spain), the University of Groningen (Netherlands), the Hebrew University of Jerusalem (Israel), the University of California, San Francisco (USA), Yale University (West Haven, USA), and Yale School of Medicine (New Haven, CT, USA).









