Protein ring discovered that coordinates “conversation” between cells
A team involving the Biofisika Institute (CSIC, EHU) uses advanced imaging techniques to observe for the first time how a protein ring guides the release of substances from inside cells.
The study reveals a key mechanism in human cells involved in processes as important as insulin secretion, neuronal communication, and tumor growth.
An international study led by Pompeu Fabra University (UPF) with the participation of the Numerical Methods of Cryo Electron Tomography Laboratory of the Biofisika Institute has succeeded in describing in unprecedented detail how cells use a ring formed by several proteins to bring together and release small “packages”—vesicles—containing hormones, proteins, or other signals necessary for the proper functioning of tissues and organs. In this research, CSIC researcher Daniel Castaño Díez, a member of the Biofisika Institute, has played a leading role in the structural characterization of this mechanism.
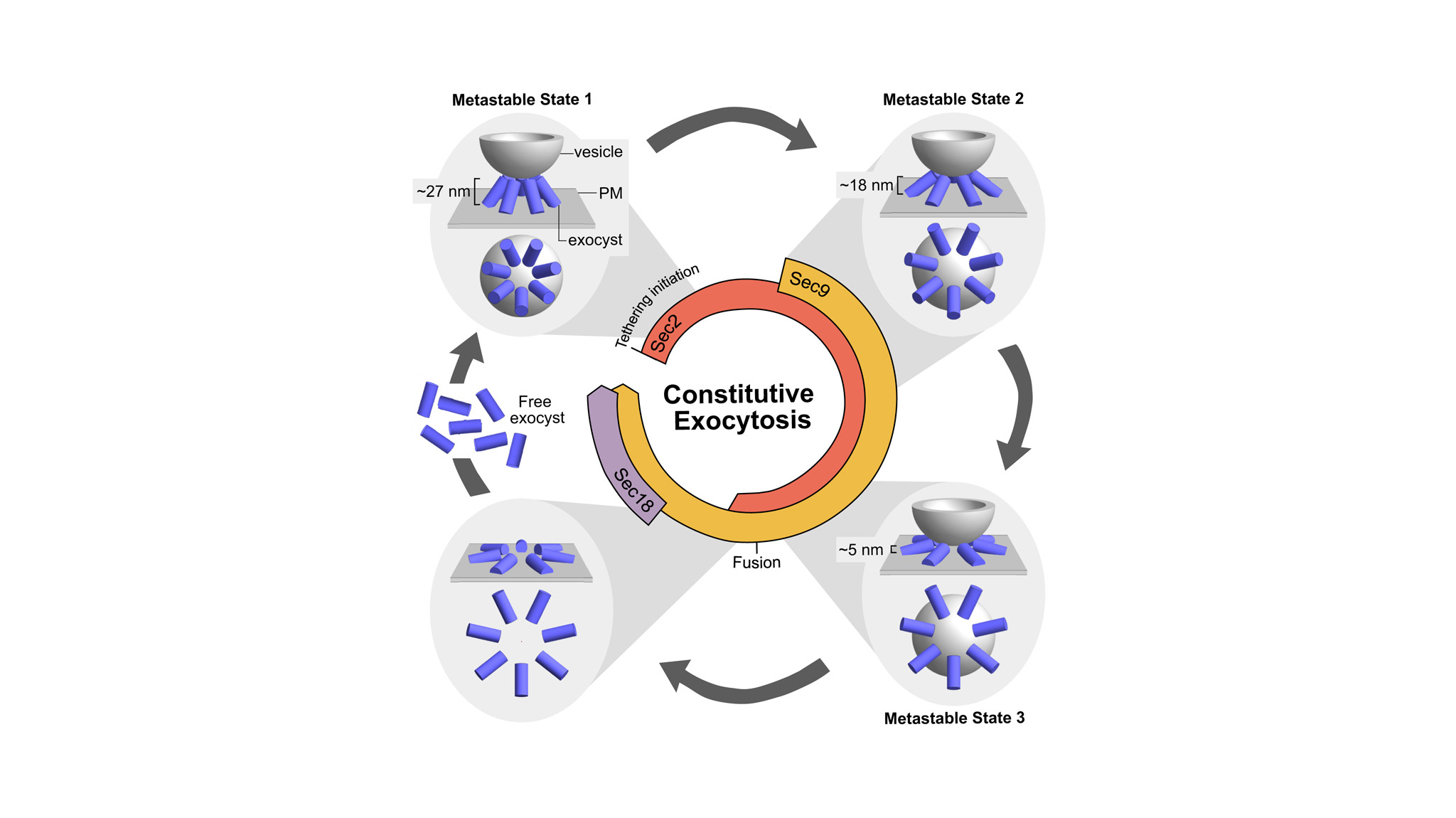
All the cells in our body constantly send and receive information, and they do so using vesicles that transport essential substances. This new study shows that several proteins assemble to form a flexible ring that helps bring these vesicles closer to the cell surface so that they can release their contents. Although the involvement of these proteins was already known, until now it had not been observed exactly how they organize themselves or how they change during the process. “What we have seen is a highly precise choreography: the ring guides the vesicle, controls the distance to the membrane, and sets the pace of the process,” explains Castaño Díez. “Having this level of detail helps us better understand how cells communicate and what can go wrong in different diseases.”
To reconstruct this mechanism, the team combined several advanced imaging technologies. Super-resolution microscopy allowed them to observe structures within living cells in extraordinary detail, while cryo-electron tomography froze the cells almost instantly to preserve their natural structure and obtain very high-resolution three-dimensional images. “By combining these new methodologies, we have been able to see a fundamental and vital cellular process that, due to its short lifespan and dynamism, was very difficult to capture,” says Oriol Gallego of the UPF.
In addition, advanced computational analysis—an area in which the contribution of the Biofisika Institute, through the team led by Raffaele Coray and Andrés Molina, has been essential—has made it possible to integrate all this information. Thanks to the combination of these techniques, it has been observed how seven copies of the protein complex assemble in a ring shape around the vesicle and how this ring changes shape as the vesicle moves toward the membrane.
The study reveals that vesicle approximation does not occur continuously, but rather in three successive stages. First, the ring forms when the vesicle is still at a certain distance; then, the ring expands and the vesicle gradually approaches; and finally, the vesicle reaches the proximity necessary to fuse with the membrane and release its contents. After this fusion, the ring disassembles to allow its components to participate in new rounds of cellular communication.
The work also identifies the fundamental role of a protein called Sec18, which acts as a kind of “reset key.” This molecule is necessary to disassemble the ring once the vesicle has released its contents. When Sec18 does not function properly, the ring remains assembled for too long and the cell cannot prepare new vesicles as efficiently, slowing down the cell communication process.
Although the study was conducted on yeast, the principles observed are very similar in more complex organisms, including humans. The machinery that moves and releases vesicles is involved in processes as important as insulin secretion in the pancreas, communication between neurons, and the release of substances that promote tumor growth and expansion.
Understanding how this protein ring works and how its disassembly is regulated thus helps lay the groundwork for investigating what happens when this process fails in human diseases.
Alongside the Biofisika Institute (CSIC,EHU), laboratories led by Oriol Gallego (Pompeu Fabra University), Carlo Manzo (University of Vic – Central University of Catalonia), Jonas Ries (Max Perutz Labs, University of Vienna), and Alex de Marco (New York Structural Biology Center), among other institutions such as EMBL Heidelberg and the University of Barcelona.









