New Preprint Reveals Mitochondrial Proteostasis Mechanisms Using Cryo-EM Data from BREM
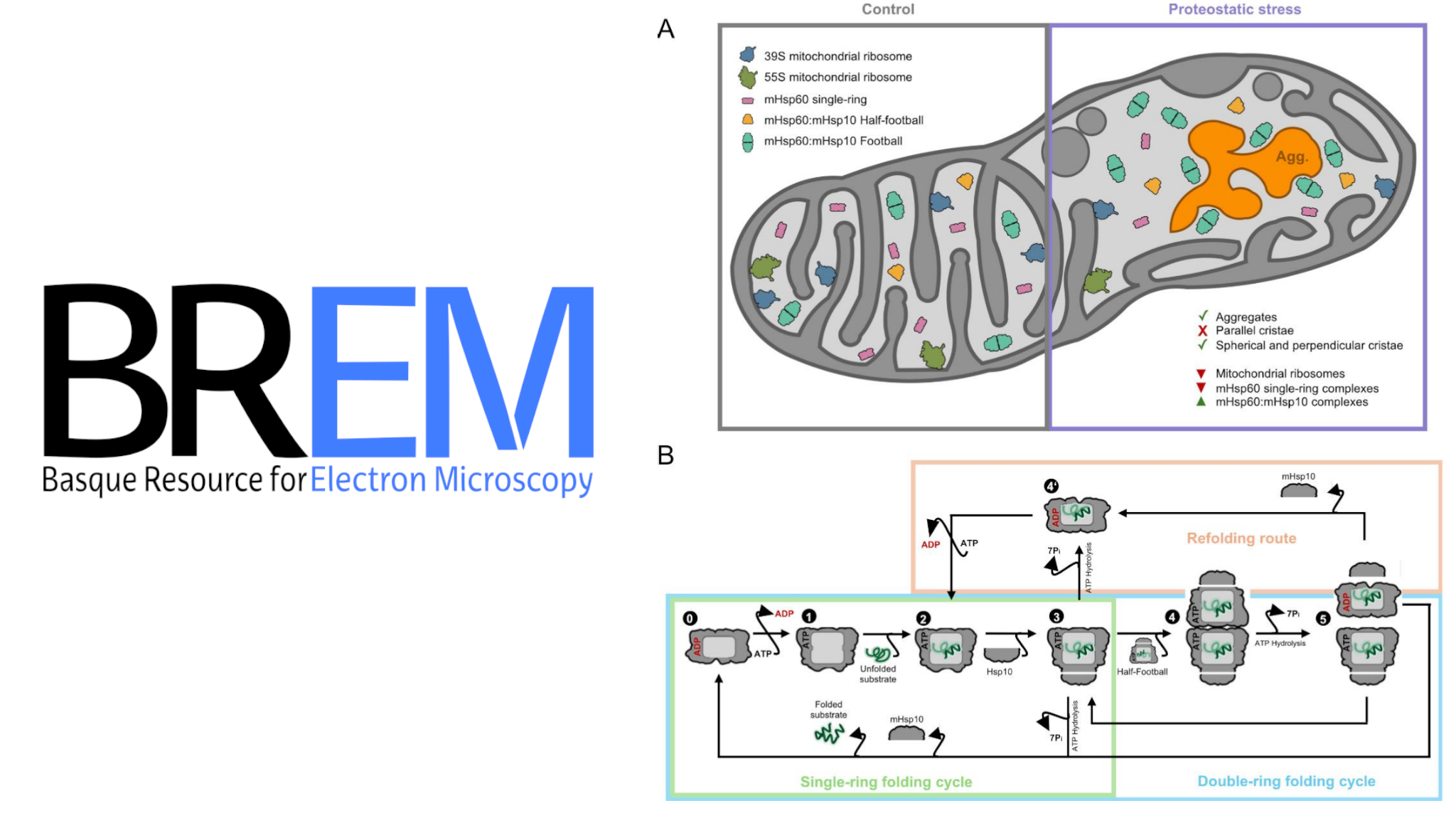
A new preprint on bioRxiv, authored by researchers from Ruben Busnadiego’s group at the University of Göttingen and Iban Ubarretxena’s lab at the Biofisika Institute, sheds light on how mitochondria adapt to proteostatic stress. Using cryo-electron tomography, the team visualized dramatic changes in mitochondrial architecture and protein synthesis machinery under stress conditions.
Their observations revealed the formation of protein aggregates in the mitochondrial matrix, significant alterations in cristae organization, and a marked decrease in mitochondrial ribosomes. Notably, they captured structural transitions of the chaperonin mHsp60 and its co-chaperone mHsp10, enabling the construction of a detailed model of the mitochondrial protein-folding cycle.
These findings provide new insights into the structural mechanisms that support mitochondrial protein homeostasis and the organelle’s ability to reprogram its biogenesis pathways in response to stress.
The single-particle cryo-EM data used in this study were collected at the Basque Resource for Electron Microscopy (BREM), part of the Instituto Biofisika.









